By
María Goretti Llamas-Arriba, Annel M. Hernández-Alcántara, Mari Luz Mohedano, Rosana Chiva, Lorena Celador-Lera, Encarnación Velázquez, Alicia Prieto, María Teresa Dueñas, Mercedes Tamame, Paloma López.
Received, 5 July, 2021 / Revised, 14 August 2021.
Accepted: 24 August
2021.
Published, 26 August 2021.
(This article belongs to the Special Issue Functional Analysis of Lactic Acid Bacteria and Bifidobacteria and Their Effects on Human Health).
Academic Editor: Arun K. Bhunia.
Abstract
Many lactic acid bacteria (LAB) produce metabolites with applications in the food industry, such as dextran-type exopolysaccharides (EPS) and riboflavin (vitamin B2).
Here, 72 bacteria were isolated from sourdoughs made by Spanish bread-makers. In the presence of sucrose, colonies of 22 isolates showed a ropy phenotype, and NMR analysis of their EPS supported that 21 of them were dextran producers.
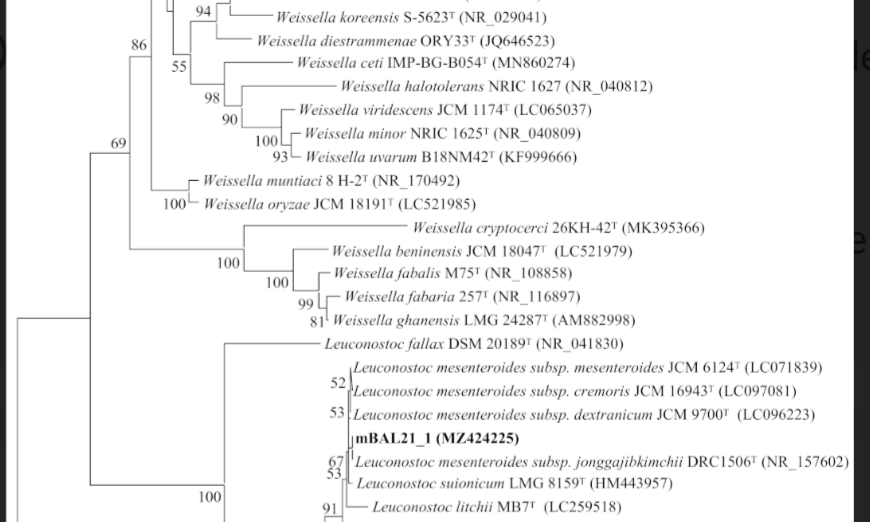
These isolates were identified by their random amplified polymorphic DNA (RAPD) patterns and their rrs and pheS gene sequences as LAB belonging to four species (Weissella cibaria, Leuconostoc citreum, Leuconostoc falkenbergense and Leuconostoc mesenteroides).
Six selected strains from the Leuconostoc and Weissella (3) genera grew in the absence of riboflavin and synthesized vitamin B2. The EPS produced by these strains were characterized as dextrans by physicochemical analysis, and the L. citreum polymer showed an unusually high degree of branching.
Quantification of the riboflavin and the EPS productions showed that the W. cibaria strains produce the highest levels (585–685 μg/and 6.5–7.4 g/L, respectively).
Therefore, these new LAB strains would be good candidates for the development of fermented foods bio-fortified with both dextrans and riboflavin. Moreover, this is the first report of riboflavin and dextran production by L. falkenbergense.
Introduction.
Lactic acid bacteria (LAB) occur naturally in fermented foods, such as “mother doughs” (also named Type I sourdoughs) or bakery doughs from bakers’ stores. The microorganisms and their metabolites isolated from these fermented matrices are classified as qualified presumption of safety (QPS), generally regarded as safe (GRAS), and considered as non-toxic and food-grade microorganisms.
Spontaneous sourdoughs inoculated into only flour and water are fermented by plant and/or cereal matrix-associated microbiota. The most prevalent initial bacterial species belong to the family Enterobacteriaceae, which are later replaced by more acid-tolerant Weissella and Leuconostoc species, until a number of different lactobacilli prevail.
The metabolic transformations carried out by LAB are potentially an important biotechnological process for the functionalization and/or nutritional fortification of breads. Thus, great attention is given to the discovery and characterization of new LAB strains that are able to biosynthesize postbiotics such as exopolysaccharides (EPS) to exploit their functional properties in foods, especially in bread.
Many LAB strains of different species produce EPS, especially dextrans with a linear backbone mainly composed of D-glucopyranosyl residues with α-(1→6) linkages and varying percentages of α-(1→4), α-(1→3), or α-(1→2) branches, which can act as immunomodulators or antivirals. In addition, the EPS produced in situ by some LAB species are of interest because of their contribution to the rheology of the doughs and to the texture of the products, especially in gluten-free breads.
Apart from dextrans, LAB can also be a valuable source for vitamin biofortification of sourdoughs. Some of these vitamins are found as precursors of intracellular co-enzymes (e.g., riboflavin (vitamin B2) and precursors of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD)), which are necessary for the regulation of biochemical reactions (oxidation–reduction) in the cell. Currently, thirteen vitamins have been recognized as being essential to human health: fat-soluble vitamins (A, D, E, and K) and water-soluble (vitamin C and B-group vitamins). As humans are not capable of synthesizing most vitamins, they have to be obtained exogenously.
B-group vitamins (thiamine, riboflavin, niacin, pyridoxine, pantothenic acid, biotin, folate, and cobalamin) differ chemically but act in synergy to maintain the body’s homeostasis.
Nowadays, B vitamin deficiencies occur as a result of non-balanced and unvaried diets, mainly in developing countries, whose populations’ staple foods are cereals and pseudo-cereals. In addition, processing and cooking of the cereals increases the loss of a large portion of B-group vitamins, such as vitamin B2, folates, or thiamine. Therefore, many countries have adopted mandatory fortification programs with specific vitamins and minerals. Due to the above, the use of vitamin-producing microorganisms could be considered a cost-effective method to produce fermented foods with high concentrations of naturally synthesized vitamins and thereby avoid the undesirable side effects associated with chemically synthesized vitamins that are used in the fortification of food.
Some LAB species such as Lactococcus lactis, Lactobacillus gasseri, and Lactobacillus reuteri, as well as Bifidobacterium adolescentis are able to produce B vitamins in high quantities in fermented foods, and various other strains isolated from a wide variety of niches were successfully employed to improve the B-group vitamins content of foods.
Recently, riboflavin fortification using selected riboflavin-producing LAB strains in food matrices such as milk, soymilk, whey, kefir-like cereal-based beverages, and pseudo-cereals was reported.
Additionally, bread and pasta are emerging as functional foods, because their characteristics allow them to be enriched in vitamins]. Therefore, the use of riboflavin-producing LAB to bio-enrich foods represents a more natural and consumer-friendly alternative to the use of chemically synthesized pseudo-vitamins.
In this context, this work is focused on the search, isolation from different sourdoughs, and characterization of dextran and riboflavin-producing LAB, with the future aim of formulating defined microbial consortia to produce functional bio-fortified breads.
Materials and Methods
Bacterial Isolation
Seventy-two LAB isolates were recovered from samples provided by various bread-makers. Sixty-four strains were recovered from mother doughs (MD) obtained by spontaneous fermentations and back-slopping procedures: 45 from MD22 made of rye flour and 19 from MD21 made of wheat flour. Moreover, 8 were recovered from a bakery dough (BD16).
The LAB strains recovered from MD21 and BD16 [30] belonged to the shared “PANBAL” collection (IBFG-CSIC Institute and the University of Salamanca, Salamanca, Spain) and those from MD22 belonged to the IBFG-CSIC collection. Of these, 19 from MD22, 1 from MD21, and 2 from BD16 (Table S1) showed a ropy phenotype in MRS medium (Man, Rogosa and Sharpe medium, Sigma Co, Darmstadt, Germany) supplemented with 5% sucrose, and they were studied in this work.
For LAB isolation, samples of 10 g of each sourdough were homogenized in 90 mL of sterile peptone water (1 g/L peptone, 8.5 g/L NaCl) in 250 mL flasks and incubated at 28 °C for 1 h with shaking (200 rpm). Then, 5 mL samples were centrifuged (3000× g), the supernatants were diluted 10-fold, and 0.1 mL aliquots were spread on MRS-agar plates containing 2% glucose (MRSG-agar).
The plates were incubated at 28 °C for 4 days. Individual colonies were transferred to MRS-agar plates containing 5% sucrose to detect the ropy phenotypes, which were classified as strong, normal, and light on the basis of their mucous appearance on this medium.
DNA Extraction and Random Amplified Polymorphic DNA (RAPD) Pattern Analysis
For RAPD fingerprinting, the total DNA was extracted from bacterial cells grown on MRSG-agar plates for 48 h at 28 °C, as described by Rivas et al. Using the total DNA as a template, the RAPD patterns were obtained by the Polymerase Chain Reaction (PCR) using the M13 primer (5′-GAGGGTGGCGGTTCT-3′) and Dream Taq-polymerase (Thermo Fisher, Madrid, Spain). PCR conditions were as follows: preheating at 95 °C for 10 min, 39 cycles of denaturing at 94 °C for 1 min, annealing at 45 °C for 1 min, extension at 72 °C for 2 min, and a final extension step at 72 °C for 7 min.
The PCR products were conserved at 5 °C, and 10 µL of each sample were electrophoresed on 1.5% (w/v) agarose gel in TBE buffer (100 mM Tris, 83 mM boric acid, 1 mM ethylenediaminetetraacetic acid EDTA, pH 8.5) at 6 V/cm, stained in a solution containing 0.5 μg/mL ethidium bromide, and photographed using a Gel Doc XR (Bio-Rad Laboratories, S.A., Alcobendas, Spain). (GeneRuler 1 kbp Plus DNA Ladder (Thermo Fisher Scientific Madrid, Spain) was used as a size marker.
Courtesy, thanks to MDPI.